We are always accepting resumes. You can find our existing job postings below.
JOB CODE: Lab Manager
EFFECTIVE DATE: August 1, 2016
Position PURPOSE:
The Lab Manager will be responsible for managing the day to day operations of the Lighthouse measurement services laboratory. Coordinate daily contract projects with the laboratory staff, managing, maintaining and enhancing the laboratory quality system, working with senior management to develop the measurement services business.
PRINCIPAL RESPONSIBILITIES:
· Coordinate measurement services, feasibility and other laboratory projects
· Manage laboratory staff (conduct performance evaluations, mentor, coach, and train lab staff)
- Review lab reports and documents for customer projects
· Write, maintain and control lab SOPs
· Participate in technical writing projects
· Perform experimental investigations on customer samples using Lighthouse spectrometers
ORGANIZATIONAL RELATIONSHIPS:
· Reports to President
· Interacts with Technical Support Representative, Research Scientist, Laboratory Technician, Application Engineer
CONTACTS:
· Internal: Company management and employees
· External: Customers
SUCCESS FACTORS:
- Understand analytical methods for sterile pharmaceutical applications including container closure integrity, moisture analysis and oxygen analysis
- Good laboratory and experimental practices
- Strong written, verbal and interpersonal skills
- Excellent analytical and troubleshooting skills
- Great attention to detail
- Thorough and organized with time-management skills and the ability to prioritize
- Perform well under pressure and the ability to meet deadlines
- Integrity and honesty
REQUIREMENTS:
- BS, MS or PhD Physical or Life Sciences
· 4+ years experience
- Regulated laboratory experience

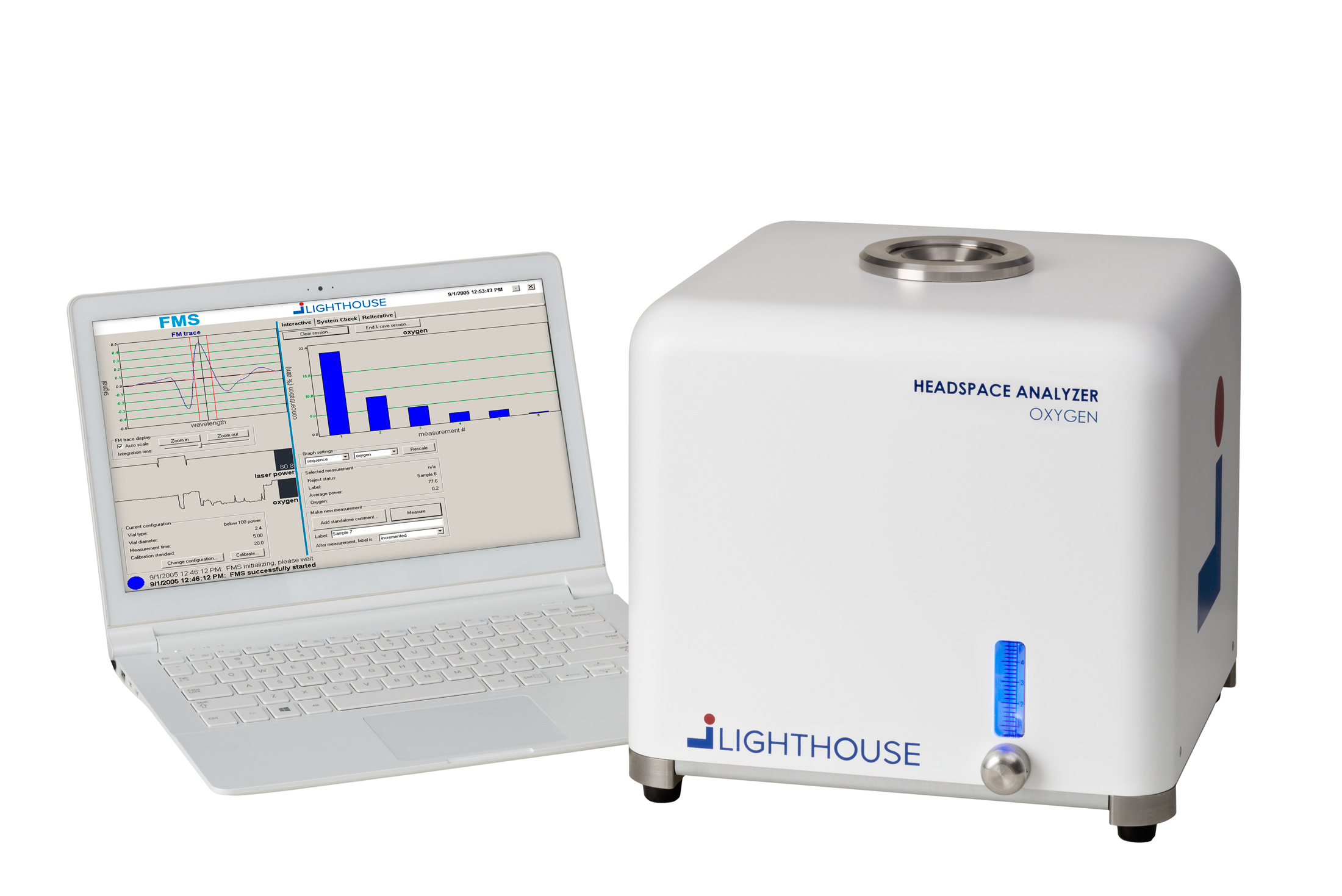 Rapid non-destructive headspace oxygen analysis
Rapid non-destructive headspace oxygen analysis